Understanding Builder TrendElectron Affinity: A Simple Guide
Are you curious about “builder trendelectron affinity“? This might sound complicated, but it’s a concept that can help explain how atoms interact with electrons. “Builder trendelectron affinity” refers to the energy change that happens when an atom gains an electron. By understanding this, we can better predict how elements will behave when they’re trying to form ions or bonds.
In this blog, we’ll take a closer look at the builder trend of electron affinity and why it’s so important in chemistry. When an element gains an electron, energy is either released or absorbed. This energy change is linked to an element’s position on the periodic table, and understanding it can help scientists predict a lot about how different atoms will react.
What is Builder Trend in Electron Affinity
The “builder trendelectron affinity” is a scientific concept that helps explain how atoms react when they gain an electron. When an atom gains an electron, it either releases or absorbs energy, and this energy change is measured by electron affinity. The “builder trend” refers to the pattern of how electron affinity changes across the periodic table.
In simple terms, the builder trend helps us predict which elements are more likely to attract electrons. This is important in understanding chemical reactions because atoms that attract electrons more easily are likely to form certain types of bonds.
How Electron Affinity Affects Chemical Reactions
When it comes to chemical reactions, the builder trendelectron affinity plays a key role. If an atom has a high electron affinity, it will attract electrons more strongly during reactions. This can help atoms form stable compounds, like salts.
Electron affinity is crucial in understanding how elements interact during chemical reactions. Elements with a high electron affinity, like the halogens, are more likely to gain electrons and form negative ions.
Why Halogens Have High Electron Affinity in the Builder Trend

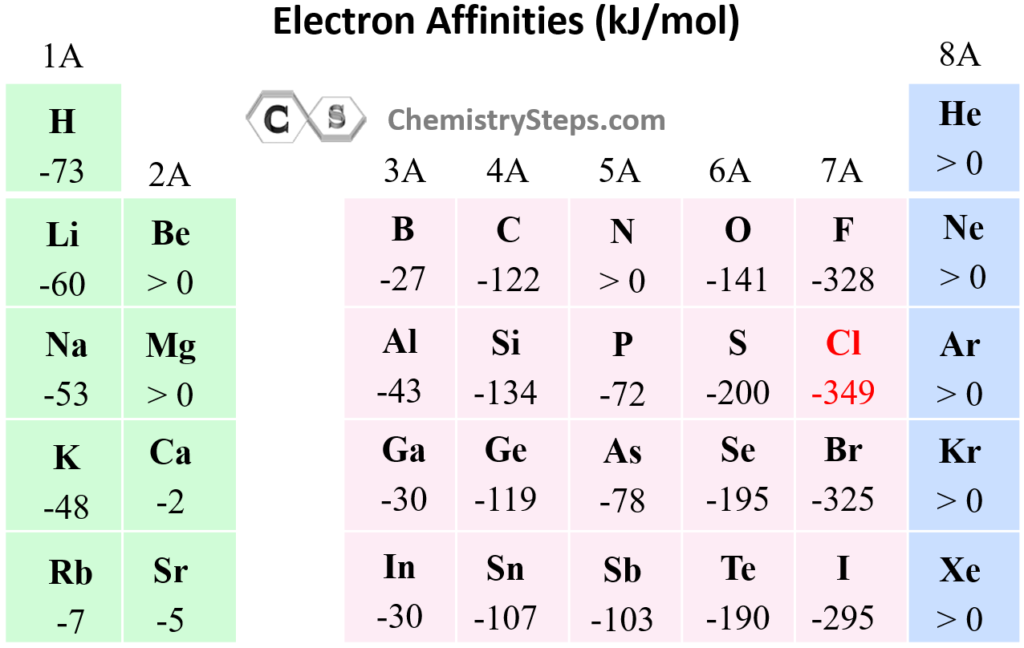
Halogens are elements in Group 17 of the periodic table, and they have some of the highest electron affinities. The builder trendelectron affinity shows that these elements are very good at attracting electrons. This is because their outer electron shell is almost full, and gaining one more electron makes them more stable. When a halogen gains an electron, energy is released, which makes the process easier for the atom.
For example, chlorine, a halogen, has a very high electron affinity. It needs just one more electron to complete its outer shell, making it more stable and less likely to react.
- Halogens have high electron affinity because they are one electron short of a full shell.
- The addition of an electron gives them the stability of a noble gas configuration.
- This high electron affinity makes halogens very reactive in chemical reactions.
This trend is important in predicting how halogens will react with other elements, and it’s why halogens are often found in compounds as negative ions.
How Builder Trend and Electron Affinity Impact the Periodic Table
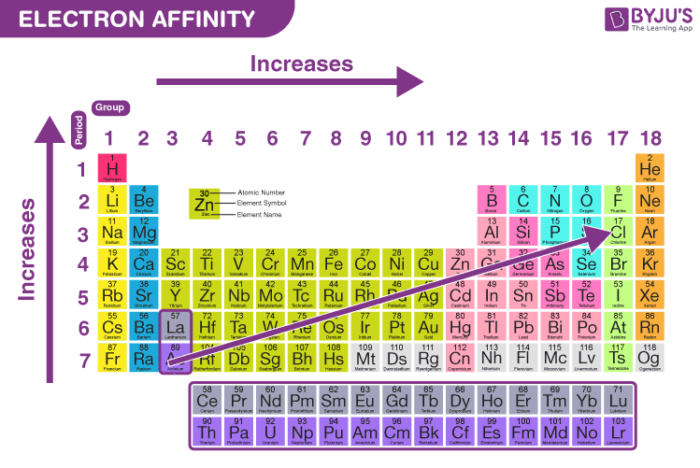
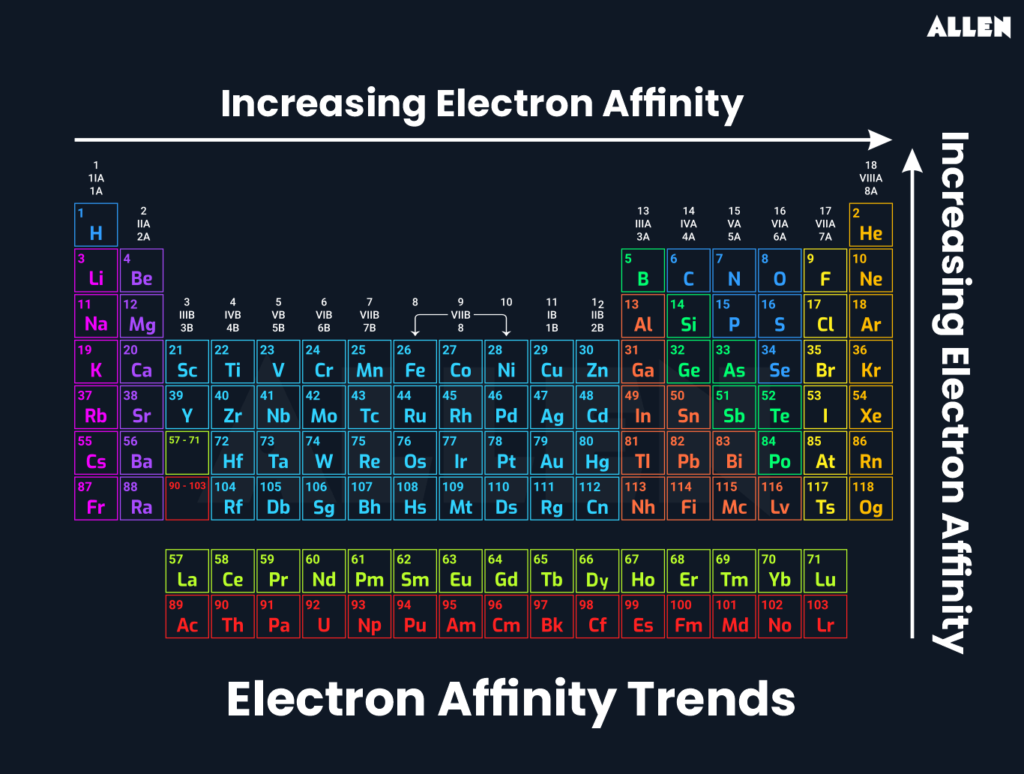
The builder trendelectron affinity is a crucial part of understanding the periodic table. As we move across a period from left to right, electron affinity generally increases, meaning elements are more likely to gain electrons and release energy. This trend helps explain the different properties of elements, such as their reactivity and their ability to form bonds.
As you move down a group on the periodic table, the electron affinity typically decreases. This happens because the outermost electrons are farther from the nucleus, so they are less strongly attracted to the nucleus.
- Electron affinity increases from left to right across a period.
- Electron affinity decreases from top to bottom in a group.
- This pattern helps explain why certain elements are more reactive than others.
Understanding these trends helps scientists predict how different elements will react in chemical reactions.
Measuring Electron Affinity: What You Need to Know
Measuring the builder trendelectron affinity can be difficult because it involves observing the energy changes when atoms gain electrons. Most of the time, electron affinities are measured for atoms in their gaseous state.
- Electron affinity is typically measured in the gaseous state.
- It’s difficult to measure accurately due to electron interactions.
- Electron affinity values are usually reported as negative numbers.
Despite these challenges, measuring electron affinity is important for understanding how atoms behave when they gain electrons.
Conclusion
In conclusion, understanding the builder trendelectron affinity helps us learn how atoms gain or lose electrons and how this affects their behavior in chemical reactions. By studying the electron affinity of different elements, we can predict how they will react and form bonds with other atoms. This is a valuable tool for scientists and chemists, especially when working with elements on the periodic table.
The builder trend also shows us important patterns, like how electron affinity increases across a period and decreases down a group. This knowledge is essential for understanding how elements like halogens behave in chemical reactions. With the right information, we can better predict how atoms will interact and make new compounds.
FAQs
Q: What is builder trendelectron affinity?
A: Builder trendelectron affinity is the energy change when an atom gains an electron. It helps explain how atoms behave in reactions.
Q: Why do halogens have high electron affinity?
A: Halogens have high electron affinity because they are just one electron short of a full outer shell, making them eager to gain an electron.
Q: How does electron affinity affect chemical reactions?
A: Electron affinity affects chemical reactions by showing how easily an atom can gain an electron and form stable compounds.
Q: Does electron affinity always increase across the periodic table?
A: Yes, electron affinity usually increases as you move from left to right across a period on the periodic table.
Q: Why is it hard to measure electron affinity?
A: It’s hard to measure electron affinity accurately because it involves observing tiny energy changes when atoms gain electrons.




